I’ve got family from Greensboro, NC visiting and we got talking about energy. They were telling me that there’s a controversy in NC over Duke Energy (a utility company) trying to charge ratepayers for a coal ash spill accident that occurred a few years ago and to pay for new regulations over coal ash. Although much of the area around Greensboro is served by Duke Energy, the city of High Point just outside of it is not. It gets its power from a different provider, with no or very little coal fired electricity and mostly nuclear power from a plant in South Carolina just southwest of Charlotte, NC. The city of High Point is totally exempt from this rate paying controversy due to nuclear. Pretty interesting! My family didn’t even know that nuclear supplies a significant portion of power to their state.
I read up on the Catawba nuclear power plant and the only incident with it listed on Wikipedia was a tritium release (I’m not saying Wikipedia is the best or only source but it’s a good source to review among many). Last year I had written an article for the New York Water Environment Association (NYWEA) on a tritium leak from the Indian Point nuclear power plant just outside of New York City. The media doesn’t do very good reporting on tritium leaks, so it reminded me of the article I wrote and that I could post it here on my website as well. This should provide context for just about any news about tritium leaks at nuclear power plants.
I hope you find this interesting and insightful. As always, I’m eager for feedback and responses!
Balancing the Facts on Tritium Levels at Indian Point
On February 6, 2016, the Entergy Corporation notified state and federal authorities and the public that elevated levels of radioactive tritium were found in 3 out of over 40 groundwater test wells underneath the Indian Point Energy Center (nuclear power plant) in Buchanan, NY, located about 30 miles north of Manhattan [1,2]. The tritium levels were approximately 1,000 times smaller than those which trigger mandatory reporting to authorities and did not pose a threat to the environment or human health, but much of the media coverage focused on alarmist angles or facts that distracted from this core message.
The timing of this incident after the water-related tragedies in Flint, Michigan and Hoosick Falls, New York might contribute to a heightened concern about water quality. Radiation is invisible and not well understood by the public or media; and, naturally, we tend to fear what we do not understand. There is also a great deal of misinformation spread about radiation by anti-nuclear organizations and individuals. It is, therefore, important to transparently discuss and analyze the radioactive tritium leak at Indian Point.
 Figure 1. The Indian Point Generating Station in Buchanan, NY
Figure 1. The Indian Point Generating Station in Buchanan, NY
Source: Wikipedia, creative commons license
What is Tritium?
Tritium is a radioactive isotope of hydrogen with a half-life of about 12 years and a biological half-life of approximately 10 days [3]. Typical non-radioactive hydrogen has 1 proton and no neutrons, while tritium has 3 nuclear particles: 1 proton and 2 neutrons. The short biological half-life of tritium means that it does not accumulate within biological organisms because half of any ingested quantity is excreted every 10 days. At 100 days after ingestion, for example, only 0.1 percent would remain (10 biological half-lives, or 0.5 raised to the power of 10).
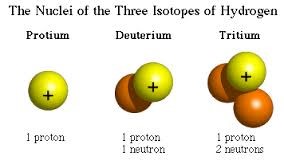 Figure 2. Nuclei of the three isotopes of hydrogen: protium (normal hydrogen), deuterium, and tritium.
Figure 2. Nuclei of the three isotopes of hydrogen: protium (normal hydrogen), deuterium, and tritium.
Source: http://education.jlab.org/glossary/isotope.html
Tritium is naturally present at very low levels in the environment via interactions of high energy cosmic rays with gaseous particles in the atmosphere. Tritium decays into a non-radioactive helium atom, releasing a beta particle which is essentially a high energy electron [3]. Radiation damages cells and DNA, but cells have elaborate repair mechanisms [4] and no health effects are observed at low doses [5].
Based on detailed studies and modeling, the Nuclear Regulatory Commission (NRC) set maximum drinking water standards for tritium at 20,000 pCi/L (picoCuries per liter of water) [6]. The Curie is a fairly large measure of radiation, but pico means one trillionth or 0.000000000001 of something, so the picoCurie – or even 20,000 pCi – represents a very small amount of radiation. The NRC’s studies showed that if water with a 20,000 pCi/L concentration of tritium was consumed by a person over the course of a whole year, this would result in a radiation dose of less than 2 mrem (millirem) in that year [6].
This 2 mrem dose is very small, and is:
** Half the dose from a roundtrip flight between Washington, DC and Los Angeles (4 mrem) (because less atmosphere at higher elevations results in lessradiation shielding).
** About 7 times less than the dose we all receive from naturally occurring radioactive potassium in our bodies (15 mrem)
** 75 times less than the average exposure Americans receive from medical tests and procedures each year (150 mrem).
** 150 times less than the average annual dose from natural background radiation in our environment (300 mrem) [6].
Thus, daily ingestion of water with a concentration of 20,000 pCi/L is safe. In fact, it could be argued that the tritium drinking water limit is rather conservative, erring on the side of caution since none of us thinks twice about the radiation dose we get from flying or from the naturally occurring radioactive atoms in our bodies.
Tritium Releases from Indian Point
The Indian Point Energy Center has two separate nuclear reactors, since unit costs are decreased by operating more than one reactor at the same site. The reactors are known as Pressurized Water Reactors (PWRs) because water at high pressures is used to cool the reactor core and carry heat to steam turbines that generate electricity. The fundamental basis of nuclear power in PWRs is that Uranium-235 absorbs a neutron, splits or fissions into 2 smaller nuclei, and this reaction releases a large amount of energy. In addition, either 2 or 3 neutrons are produced when each uranium atom fissions, and these neutrons can make other Uranium-235 atoms split. The reaction is controlled using non-fissionable materials that absorb neutrons, preventing them from reacting with U-235.
One of the ways the nuclear reaction is controlled in PWRs is by adjusting the concentration of boric acid in the coolant water. Boron is an excellent neutron absorber, giving operators a way to fine tune the power output of the reactor. The coolant water is housed in separate pipes and does not come into direct contact with the reactor. Some tritium is produced in the coolant loop by the interaction of neutrons from the reactor with boron atoms. Tritium, therefore, builds up in the coolant and must be purged from time to time. Because the concentrations are low from a safety and environmental perspective, PWR reactors are permitted to intentionally dilute and release the tritiated water under strict guidelines [6].
The elevated groundwater tritium levels beneath Indian Point indicate a leak in underground pipes that store and transport coolant water. Regulations do not allow the unintentional discharge of tritiated water no matter how low the concentration, so Indian Point must locate and fix or replace any leaky pipe sections. The best current understanding is that a leak in the pipes between storage and release is responsible for the elevated levels. Investigations into the cause continue. [1,2]
Typical concentrations of tritium in the 40 groundwater testing wells beneath Indian Point were 12,300 pCi/L. The elevated tritium levels found in three of the groundwater monitoring wells were initially as high as 8 million pCi and later increased to 14 million pCi [7,8]. Many articles reported the first figure as a dangerous or “alarming” 65,000 percent increase in radiation levels or reported that it was 400 times the drinking water limit [7]. The numerical figures themselves are accurate, but are not dangerous because the site was designed so its groundwater does not flow to drinking water, but rather flows to a discharge canal where it is intentionally diluted. In addition, further dilution to undetectable levels occurs upon reaching the Hudson River. For more information, see Reference 9, written by John Kelly, a retired radiation protection manager who oversaw construction of Indian Point and has lived near and worked at the plant for decades.
Many media articles were comparing apples to oranges by reporting radiation levels in comparison to drinking water levels. When the public reads that radiation levels are 400 times higher than regulatory limits, this naturally causes worry and sounds frightening. But the 20,000 pCi regulatory limit is specifically for drinking water and, as previously discussed, the site was specifically engineered and designed such that groundwater would be highly diluted to safe levels and would not flow into drinking water sources.
Media articles also quoted other safety related information, such as the fact that 317,000 people live within a 10-mile evacuation planning zone around the reactor [7]. This may bias the public toward thinking about catastrophic nuclear incidents, whereas, consequences of the tritium leak were insignificant from a human health or environmental perspective (but still required action to remedy).
Is Any Radiation Exposure Harmful?
One of the most common anti-nuclear arguments is that radiation is harmful at any dose. Radiation at small doses does cause limited small scale cellular damage, but the body has intricate repair mechanisms [4,5] and no long-term damage occurs. Cellular DNA can be damaged by metabolic byproducts like free radicals, and DNA copying errors are relatively frequent during cell division. “Cells have evolved a number of mechanisms to detect and repair the various types of damage that can occur to DNA, no matter whether this damage is caused by the environment or by errors in replication” [4]. We know that at low doses radiation does not produce health effects.
An example of a response to the Indian Point tritium link that focuses on the supposed danger of even a single radioactive atom is a citizens group called Shut Down Indian Point NOW!, which released the following flier to build support for a town hall meeting on the tritium leak:
 Figure 3: Flyer about shutting down Indian Point. Obtained from the “Shut Down Indian Point! NOW! Network” Facebook Page
Figure 3: Flyer about shutting down Indian Point. Obtained from the “Shut Down Indian Point! NOW! Network” Facebook Page
The flier highlights some of the misleading facts/statistics already discussed, but it also invokes images of at-risk infants and pregnant women. It further claims that the ingestion or inhalation of even a single molecule of a radioactive isotope can “cause cancer, birth defects, and mutation” — a clear case of inciting irrational fear. We continually breathe extremely low levels of radiation, such as radioactive Carbon-14 that forms the basis of carbon dating techniques. But atoms are incredibly tiny, so when we ingest or breathe extremely low concentrations of radioactive atoms we still ingest or breathe huge numbers of them!
An example is the potassium-rich banana. The average banana contains about 0.4 grams of potassium, and about 0.0117 percent of all potassium in the world consists of radioactive K-40 [10]. A single banana, therefore, contains 0.0000468 g of radioactive K-40 (0.4 g × 0.0117%). From basic chemistry, the molecular weight of potassium is 39 g/mol or 40 g/mol for K-40, and every mole contains 6.02 x 10^23 atoms (Avogadro’s number). Converting 0.000468 g of radioactive Potassium-40 to atoms, this is 7×10^17 (or 700,000,000,000,000,000) radioactive atoms ingested! From this, it should be clear that the ingestion of a single atom of a radioactive substance is not dangerous and will not cause mutations, cancer, etc.
Radiation is unseen, poorly understood by the public, and is the subject of much science fiction. Scientific knowledge and transparency surrounding topics of radiation can help alleviate public fears and help us as a society to focus our limited time and resources on issues with a high impact on human and environmental health. To use an analogy, if the tragedies in Flint, Michigan and Hoosick Falls, New York were as dangerous as charging rhinos, then the tritium leaks at Indian Point would be like a house fly – irritating, perhaps, but not a threat.
______________________________________________________________________
References
1. Entergy Statement on Comprehensive Groundwater Monitoring Program and Elevated Tritium at Indian Point, http://www.safesecurevital.com/entergy-statement-on-comprehensive-groundwater-monitoring-program-and-elevated-tritium-at-indian-point/
2. Information on Groundwater Monitoring Program and Tritium at Indian Point, http://www.safesecurevital.com/groundwater-monitoring.html
3. Idaho State University Radiation Information Network Tritium Information Section, http://www.physics.isu.edu/radinf/tritium.htm
4. Clancy, S. “DNA damage & repair: mechanisms for maintaining DNA integrity.” Nature Education 1.1 (2008): 103. http://www.nature.com/scitable/topicpage/dna-damage-repair-mechanisms-for-maintaining-dna-344
5. Nuclear Regulatory Commission. Backgrounder on Biological Effects of Radiation. http://www.nrc.gov/reading-rm/doc-collections/fact-sheets/bio-effects-radiation.html
6. Nuclear Regulatory Commission. Backgrounder on Tritium, Radiation Protection Limits, and Drinking Water Standards. http://www.nrc.gov/reading-rm/doc-collections/fact-sheets/tritium-radiation-fs.html
7. CNN. Indian Point nuclear plant leak causes radioactivity in groundwater. Feb 6, 2016. http://www.cnn.com/2016/02/06/us/nuclear-facility-ground-contamination-new-york/
8. Reuters, Indian Point tritium leak 80% worse than originally reported. Feb 10, 2016. https://www.rt.com/usa/332087-indian-point-tritium-leak/
9. Bill Kelly, Rockland Times Op-Ed. Former Entergy Manager says It’s Time to End Political Fear-Mongering about Indian Point. Feb 25, 2016. http://www.rocklandtimes.com/2016/02/25/former-entergy-manager-says-its-time-to-end-political-fear-mongering-about-indian-point/
10. Argonne National Laboratory. Human Health Fact Sheet, K-40. August 2005. http://phi.nmsu.edu/~pvs/teaching/phys593/potassium.pdf